What would happen if a chromosome failed to attach to spindle fibers?
- Commentary
- Open up Access
- Published:
Merotelic attachments and non-homologous end joining are the basis of chromosomal instability
Cell Division volume v, Article number:13 (2010) Cite this article
Abstract
Although the big bulk of solid tumors show a combination of mitotic spindle defects and chromosomal instability, little is known about the mechanisms that govern the initial steps in tumorigenesis. The recent report of spindle-induced DNA damage provides evidence for a single mechanism responsible for the most prominent genetic defects in chromosomal instability. Spindle-induced Dna impairment is brought nigh by uncorrected merotelic attachments, which cause kinetochore baloney, chromosome breakage at the centromere, and possible activation of Deoxyribonucleic acid harm repair pathways. Although merotelic attachments are common early in mitosis, some escape detection past the kinetochore pathway. Equally a effect, a proportion of merotelic attachments gives rise to chromosome breakage in normal cells and in carcinomas. An intrinsic chromosome segregation defect might thus form the basis of tumor initiation. Nosotros propose a hypothesis in which merotelic attachments and chromosome breakage establish a feedback loop that results in relaxation of the spindle checkpoint and suppression of anti-proliferative pathways, thereby promoting carcinogenesis.
Introduction
Mitosis comprises a brief period of intense activity in the jail cell cycle. The segregation of sis chromatids into daughter cells involves moving the largest molecules encountered in nature (the chromosomes) over distances greater than the size of most organelles. To ensure sufficiently rapid chromosome segregation, almost eukaryotes connect each centromere to a parcel of parallel microtubules, termed the kinetochore fiber, forth which an outward-pulling force moves sister chromatids towards the spindle poles [1]. Chromosome segregation must be completed rapidly, since mitosis represses other prison cell functions [2–4], merely accurate distribution of sister chromatids over the two girl cells is essential for the genetic integrity of the organism. Cells thus impose control on the chromosome segregation machinery through a combination of mechanisms known as the spindle checkpoint. Before chromosomes are segregated, the cell must connect each kinetochore to a single spindle pole through a unmarried kinetochore fiber (amphitelic kinetochore attachment; Fig. 1a). This is the merely situation that guarantees the fidelity of chromosome segregation, and the cell will attempt to filibuster anaphase onset if these requirements are not fulfilled. Satisfaction of the mitotic checkpoint marks a bespeak of no render, and overall chromosome movement continues in anaphase even if spindle attachments are disturbed [5, 6]; this means that spindle errors can merely exist corrected inside a express fourth dimension window, and that undetected kinetochore attachment errors tin change the genetic makeup of daughter cells.
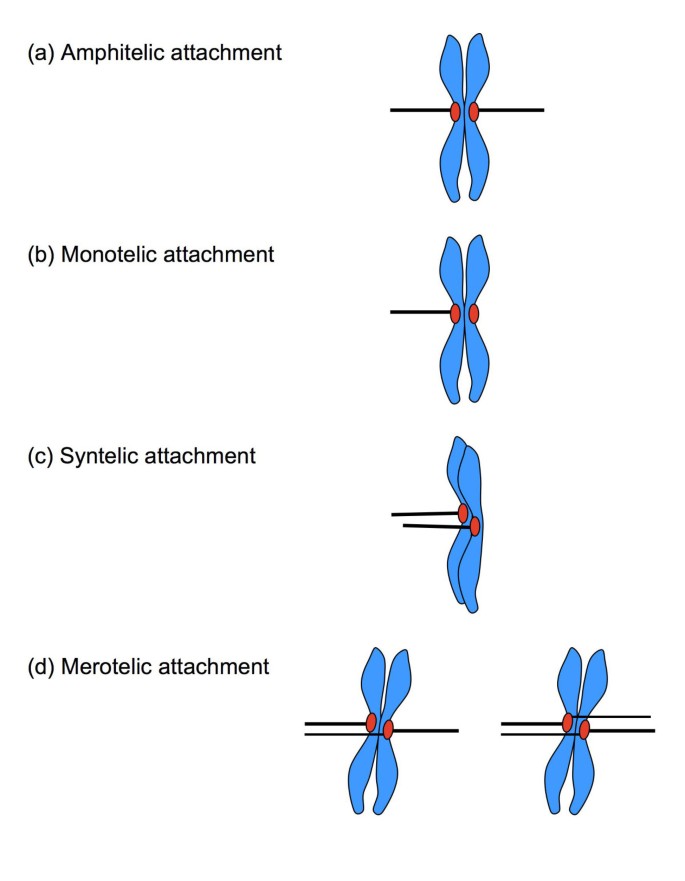
Spindle attachment defects. (a) In amphitelic zipper, the sister kinetochores are correctly connected to microtubules from reverse poles, resulting in a bioriented chromosome. (b) In a monotelic attachment, only 1 of the sis chromatids is continued to a spindle pole; the chromosome is mono-oriented. (c) In a syntelic attachment, both sister kinetochores are attached to a single spindle pole, and the chromosome is mono-oriented. (d) In a merotelic attachment, usually one or, rarely, both sister kinetochores are connected to both poles instead of i. Chromosomes are bioriented in merotelic attachments.
In addition to correct amphitelic attachment, several errors can occur in microtubule/kinetochore coupling (Fig. 1b, c, d). Individual kinetochores might not attach (monotelic attachment), and are left backside once chromosome segregation is initiated at anaphase. Kinetochores of both sister chromatids might attach to microtubules from a single spindle pole (syntelic attachment), and run the risk of segregation into the incorrect girl cell. A single kinetochore might capture microtubules from both spindle poles (merotelic zipper), which places concrete stress on the centromere as the microtubules start to pull. The offset 2 errors outcome in loss of spindle tension, are sensed as a lack of kinetochore stretch, and trigger a strong bespeak for mitotic checkpoint activation [7]. Merotelic attachments generate kinetochore tension, however, and practise not always activate the spindle checkpoint [viii–x]. Although merotelic attachments are potentially harmful, they are relatively common in dividing cells, but are usually corrected early in mitosis [xi, 12]. The control of the mitotic spindle however is deregulated in most carcinomas, resulting in a self-amplifying loop of chromosomal instability. Recent advances underline the importance of spindle defects in the early on stages of tumorigenesis, and generate a particular interest in the role of spindle-induced chromosome breakage as the initiator of chromosomal instability [thirteen]. The aim of this paper is to discuss some of the signaling pathways that connect spindle defects, specifically merotelic attachments, to chromosome breakage and the regulation of prison cell cycle progression.
Coping with merotelic attachments
Uncorrected merotelic attachments pb to gains and losses of whole chromosomes, termed aneuploidy [eleven]. In add-on, uncorrected merotelic attachments tin can exert sufficient forcefulness to misconstrue individual kinetochores, which amercement centromeric chromatin and causes chromosome rupture [13]. The alterations that result from uncorrected merotelic attachments (aneuploidy as well equally losses and gains of chromosome arms) are amid the most frequently observed genomic defects in cancer [xiv, xv]. Since uncorrected merotelic attachments appear to exist common in solid tumors, thery are thought to be a driving strength backside the chromosomal instability (CIN) phenotype that accounts for approximately 85% of sporadic carcinomas [sixteen, 17]. The chromosome breakage that is associated with uncorrected merotelic attachments generates "reactive" chromosome arms that are able to fuse to intact chromosomes [17]. Such "reactive" artillery could initiate the self-propagating concatenation of instability termed the breakage-fusion-bridge wheel [eighteen]. Whereas the Dna breakage products of uncorrected merotelic attachments, whole chromosome arms, are specially mutual in low-grade tumors, complex translocation patterns are feature of high-grade carcinomas [19, 20]. In CIN tumors, uncorrected merotelic attachments might thus initiate genomic instability that is subsequently propagated past breakage-fusion-bridge cycles [17]. Although uncorrected merotelic attachments are common in CIN tumors that prove reduced spindle checkpoint control, some good for you cells likewise bear spindle defects. Genetic techniques using fluorescent probes that flank the centromere showed that a minor proportion of normal lymphocytes undergo physical separation of the long and short arms of a unmarried chromosome [21], indicating that some merotelic attachments lead inevitably to chromosome breakage. The uncorrected merotelic attachments responsible for the virtually important genomic alterations of CIN tumors thus occur occasionally in normal cells.
The prevalence of CIN in cancer and the evidence of uncorrected merotelic attachments in normal cells advise that correct chromosome segregation is a fundamental problem in development, however not fully resolved. Some species, for example Muntiacus muntjak, Potorous tridactylis, and Wallabia bicolor [22–24], get together their genome in a dozen or fewer chromosomes, with a concomitant reduction in centrosome number. Although low chromosome numbers reduce the number of kinetochores that require control in each cell partition, individual kinetochores still class merotelic attachments in Potorous tridactylis cells [25]. An extremely low chromosome number nonetheless appears to foreclose aneuploidy, thought to be i of the initiating events in tumorigenesis [16, 26]. Weather condition that readily induce aneuploidy in human and mouse cells but allow for loss or gain of the small sex chromosome Y2 in muntjac cells. Missegregation of the big chromosomes in muntjac is not tolerated due to cistron dosage effects [27]. Near mammals must live with the occasional aneuploid cell, yet, because they fully depend on spindle dynamics to detect and forestall chromosome missegregation [12, 25].
Since the classical mitotic checkpoint fails to detect a proportion of merotelic attachments [viii–x], a backup mechanism that detects the consequences of uncorrected merotelic attachments and prevents continuation of mitosis could provide a solution. In improver to aneuploidy, uncorrected merotelic attachments generate chromosome fragments, that is, the formation of double-strand breaks (DSB). DSB could thus indicate a chromosome segregation trouble to the cell. Intramitotic Deoxyribonucleic acid damage indeed produces an anaphase filibuster point; mammalian cells observe mitotic DNA breaks and respond by activating the spindle checkpoint [28–30]. The crosstalk between break repair and spindle control pathways might have a physiological function in the prevention of aneuploidy, since treatments that induce DNA harm cause aneuploidy in normal cells [31–33]. Although identification of damaged DNA seems a second-best solution, coupling DSB detection to anaphase filibuster serves the dual purpose of creating a fourth dimension window for repair and reattaching spindle fibers to the kinetochore (Fig. ii). The state of affairs is more than circuitous in carcinomas that bear witness a weakened response to anaphase delay signals, termed mitotic slippage [16, 26]. Mitotic slippage and alterations in the master detection of kinetochore zipper defects would increase the number of DSB, adding pressure to the detection and repair pathway. Although the intermission repair pathway might be activated by uncorrected merotelic attachments and the associated Deoxyribonucleic acid impairment, it would exist ineffective in mitosis if a downstream anaphase delay signal is dumb or bypassed.

Signaling by spindle attachment defects. Two independent pathways act to filibuster anaphase. The spindle zipper pathway senses kinetochore tension and is especially efficient for detecting monotelic and syntelic attachments, and the DNA impairment pathway acts as an boosted mechanism that responds to DSB generated by merotelic attachments. When kinetochore attachment defects are undetected, for example in tumors with a CIN phenotype (grey), merotelic attachments and DSB increase, leading to activation of the DNA harm pathway and ultimately to mitotic slippage.
How cells handle chromosome breaks in mitosis
Although merotelic attachments are processed past diverse pathways, a small proportion escapes detection [21], leaving the daughter cells to deal with a fragmented chromosome. Relaxation of the spindle checkpoint exacerbates this trouble [13], placing additional force per unit area on Deoxyribonucleic acid intermission repair in CIN tumors. In mammalian cells, double-strand breaks are repaired by two major processes, termed non-homologous terminate joining and homologous recombination [34]. The availability of repair pathways at the time and subcellular location of intra-mitotic DSB has important consequences; whereas non-homologous terminate joining repairs breaks past simple religation of two DNA ends, homologous recombination depends on a homologous Dna template. This means that non-homologous finish joining can repair DSB throughout the prison cell cycle, but homologous recombination is nearly inactive in the G1 phase [35]. The Deoxyribonucleic acid breaks caused by uncorrected merotelic attachments are physically the same as other DSB and their centromeric location does non in itself hinder efficient repair [36], but the cell wheel stage in which they are formed obliges the cell to correct Deoxyribonucleic acid impairment during or right after mitosis. In addition, some chromosome fragments are sequestered in micronuclei [13], resulting in physical separation from the remainder of chromosomes and precluding homologous recombination.
Mice deficient in any of the DSB repair proteins are generally hypersensitive to induced Dna damage, although they are usually viable [37, 38]. Whereas not-homologous end joining or homologous recombination repair mutants have problems repairing induced DSB, the inactivation of a single repair pathway does non result in spontaneous DSB accumulation [13, 39, twoscore]. The absence of spontaneous Dna damage in mice defective a single repair pathway implies that the endogenous DSB formation rate must be relatively low or at least is not life threatening. Notwithstanding the low frequency of spontaneous DSB, many tumors evidence increased repair system activeness, in particular that of non-homologous finish joining [41–43]. Non-homologous end joining activation in cancer indicates that DSB are generated at an increased rate, possibly due to chromosome segregation errors and concomitant chromosome arm breakage.
Not-homologous end joining is essential in a CIN background
Non-homologous terminate joining appears to be especially important when spindle checkpoint command is relaxed, because the increase in uncorrected merotelic attachments could promote chromosome breakage. In non-homologous end joining, Ku80 is essential for recruitment of repair complexes to DSB, whereas DNA-PKcs is the principal repair kinase [44]. Although residuum non-homologous terminate joining takes place in both Ku80- and DNA-PKcs-deficient cells, Ku80 mutation has a far greater touch on on DSB repair kinetics than DNA-PKcs mutation [45–47]; Deoxyribonucleic acid-PKcs disruption thus produces a milder phenotype than Ku80 inactivation. Targeted disruption of the death inducer obliterator (Dido) gene, which causes centrosome amplification and spindle checkpoint relaxation [48], results in a CIN phenotype that includes aneuploidy and chromosome breakage [xiii]. To determine whether non-homologous finish joining is essential in a CIN background, we crossed Dido and Ku80 heterozygous mice, interbred the double heterozygotes and genotyped all offspring. Dido and Deoxyribonucleic acid-PKcs heterozygous mice were interbred in the aforementioned way. In our crosses, heterozygous and wild-type pups were born at frequencies compatible with normal Mendelian inheritance; we found slightly fewer Ku80 and Dido mutant newborns (Tabular array 1). In over 1000 pups tested, however, we identified no Dido Ku80 double mutants. When double heterozygous Dido Deoxyribonucleic acid-PKcs mice were crossed, Dido mutants and Dido DNA-PKcs double mutants were built-in at frequencies beneath the expected ratio, only no marked effect of DNA-PKcs mutation was found (Table 2). Although the frequency of Ku80 mutants was reduced, some Dido Ku80 double mutants would be expected; the absence of these double mutant mice thus indicates synthetic lethality, in accordance with the reported intra-mitotic DSB in the Dido mutant [13]. Mutation of Ku80 has a far greater impact on DSB repair kinetics than that of DNA-PKcs in models of induced DNA damage [49, 50], and DNA-PKcs besides appears to exist less important than Ku80 in the repair of DSB generated by uncorrected merotelic attachments.
Since Dido Ku80 double mutant embryos die in utero, nosotros established the time of gestation at which death occurs. Double heterozygous Dido Ku80 mice were interbred and embryos analyzed by dark field microscopy at diverse times postcoitum. Mutant embryo evolution was not markedly different from that of heterozygous counterparts up to E8.five (not shown). Growth filibuster in Dido Ku80 double mutant embryos was beginning credible at E9.5, with underdeveloped head, heart and somites (Fig. 3). At E10.five, Dido and Ku80 single mutant embryos continued to develop unremarkably, whereas most Dido Ku80 double mutant embryos had died and were being reabsorbed, and none survived beyond E12.five. Due to variation in survival, we were unable to define an verbal time point of death. These data nonetheless bear witness that Dido Ku80 double mutant embryos die in utero at mid-gestation, suggesting a role for not-homologous finish joining in the repair of Dna damage generated by uncorrected merotelic attachments.
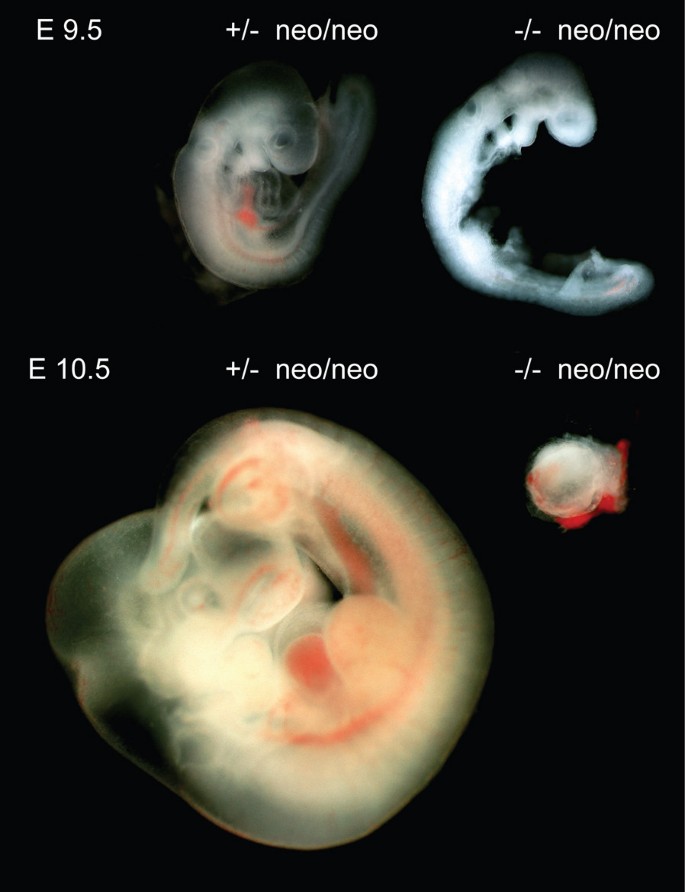
Combined disruption of Ku80 and Dido is lethal in mid-gestation. The figure shows embryos isolated at embryonic day E9.v (top) and E10.5 (bottom). Ku80 heterozygous Dido mutant embryos are shown at left and Ku80 Dido double mutant embryos at correct. At E9.5, double mutant embryos show growth delay in head, heart, and somites. At E10.v, most Ku80 Dido double mutant embryos are being resorbed. Magnification, twoscore-fold. All animal experiments were performed in compliance with EU and CNB animate being committee directives.
Endmost remarks
Merotelic kinetochore attachments seem to be the Achilles' heel of mammalian cell division, as they tin can bring most potentially dangerous genomic instability just are poorly recognized by the spindle checkpoint. Even in normal cells, a minor proportion of cell divisions thus requite ascent to chromosome breakage [21]. In the case of intramitotic chromosome breakage, DSB repair systems could transmit a 2d signal in an effort to delay mitosis progression [28–30]. The combination of signals involved in the detection of spindle errors has important consequences for cancer development, and gives ascent to a working model of early on tumorigenesis (Fig. 4).
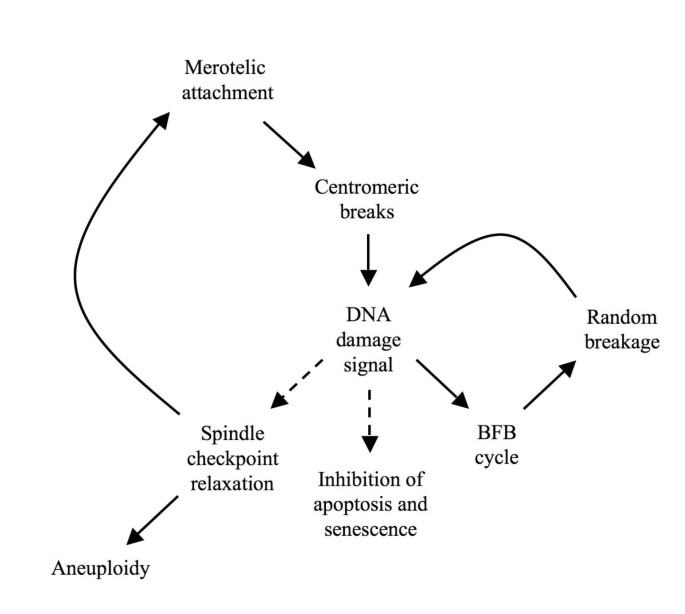
Model for amplification of chromosomal instability by Dna damage. Initial merotelic attachments activate DNA harm signaling and inhibit prison cell proliferation through anaphase filibuster and induction of apoptosis or senescence. To overcome the block, the downstream spindle checkpoint is suppressed in CIN tumors, increasing the frequency of spindle zipper errors. Equally a side issue of this continuous breakage, Dna repair mechanisms remain activated, leading ultimately to adaptation through suppression of apoptosis and senescence and through spindle checkpoint relaxation (dashed lines).
Any minor alteration in spindle regulation could event in an increase in merotelic attachments that escape detection, giving ascent to aneuploidy and chromosome breakage [xiii]. Breakage activates cellular Deoxyribonucleic acid damage command, shown by increased DSB repair in many tumors [41–43]. The need for non-homologous stop joining in a CIN groundwork is emphasized past the synthetic lethality of Dido Ku80 double mutants. DNA harm signaling provides feedback to the spindle checkpoint and delays mitosis progression, which prolongs the time window for repair and prevents aneuploidy. Repair by non-homologous cease joining not but limits Dna damage and promotes cell survival, only also catalyzes the fusion of reactive chromosome ends. A chromosome fragment generated past spindle defects can thus form end-to-end fusions with normal chromosomes and initiate the breakage-fusion-span cycle [18]. Once the breakage-fusion-span cycles commence, restoring spindle command no longer ensures stability, since dicentric chromosomes formed past end-to-cease fusions can break, even though private kinetochores are correctly attached [17, eighteen]. A long term effect of Dna damage is cell immortalization; sustained breaks exert selective pressure to evade apoptosis and senescence [51]. Since DSB prevent the progression of mitosis, it is probable that sustained breaks besides facilitate mitotic checkpoint relaxation. Continuous mitotic chromosome breakage could thus explain why, over time, CIN tumors become more cancerous and refractory to treatment. In conclusion, nature's use of DSB repair systems as a fill-in for the detection of merotelic attachments might in fact promote chromosomal instability and act as a motor for carcinogenesis. CIN tumors show precisely the characteristics predicted by the above model: Nigh carcinomas testify chromosomal instability and reduced command of the mitotic spindle, combined with enhanced DNA damage repair and reduced apoptotic potential. The claiming for cancer treatment will be to break this savage circle without causing additional genomic instability.
Abbreviations
- CIN:
-
chromosomal instability
- DSB:
-
double strand Deoxyribonucleic acid break.
References
-
Maiato H, Sunkel CE: Kinetochore-microtubule interactions during cell division. Chromosome Res 2004, 12: 585–597. 10.1023/B:CHRO.0000036587.26566.81
-
Berlin RD, Oliver JM, Walter RJ: Surface functions during Mitosis I: phagocytosis, pinocytosis and mobility of surface-bound Con A. Prison cell 1978, 15: 327–341. 10.1016/0092-8674(78)90002-8
-
Klein J, Grummt I: Cell cycle-dependent regulation of RNA polymerase I transcription: the nucleolar transcription factor UBF is inactive in mitosis and early on G1. Proc Natl Acad Sci Us 1999, 96: 6096–6101. 10.1073/pnas.96.11.6096
-
Gottesfeld JM, Forbes DJ: Mitotic repression of the transcriptional machinery. Trends Biochem Sci 1997, 22: 197–202. 10.1016/S0968-0004(97)01045-i
-
Simerly C, Balczon R, Brinkley BR, Schatten Chiliad: Microinjected centromere kinetochore antibodies interfere with chromosome movement in meiotic and mitotic mouse oocytes. J Cell Biol 1990, 111: 1491–1504. 10.1083/jcb.111.four.1491
-
Wise DA, Bhattacharjee L: Antikinetochore antibodies interfere with prometaphase but not anaphase chromosome movement in living PtK2 cells. Cell Motil Cytoskeleton 1992, 23: 157–167. 10.1002/cm.970230208
-
Maresca TJ, Salmon ED: Welcome to a new kind of tension: translating kinetochore mechanics into a await-anaphase point. J Cell Sci 2010, 123: 825–835. x.1242/jcs.064790
-
Cimini D, Fioravanti D, Salmon ED, Degrassi F: Merotelic kinetochore orientation versus chromosome mono-orientation in the origin of lagging chromosomes in human primary cells. J Cell Sci 2002, 115: 507–515.
-
Lampson MA, Renduchitala K, Khodjakov A, Kapoor TM: Correcting improper chromosome-spindle attachments during cell division. Nat Cell Biol 2004, half dozen: 232–237. 10.1038/ncb1102
-
Cimini D, Wan Ten, Hirel CB, Salmon ED: Aurora kinase promotes turnover of kinetochore microtubules to reduce chromosome segregation errors. Curr Biol 2006, 16: 1711–1718. x.1016/j.cub.2006.07.022
-
Cimini D, Howell B, Maddox P, Khodjakov A, Degrassi F, Salmon ED: Merotelic kinetochore orientation is a major mechanism of aneuploidy in mitotic mammalian tissue cells. J Prison cell Biol 2001, 153: 517–527. 10.1083/jcb.153.iii.517
-
Cimini D, Moree B, Canman JC, Salmon ED: Merotelic kinetochore orientation occurs often during early mitosis in mammalian tissue cells and mistake correction is achieved by 2 dissimilar mechanisms. J Cell Sci 2003, 116: 4213–4225. 10.1242/jcs.00716
-
Alonso Guerrero A, Cano Gamero M, Trachana 5, Futterer A, Pacios-Bras C, Panadero Diaz-Concha N, Cigudosa JC, Martínez-A C, van Wely KH: Centromere-localized breaks bespeak the generation of DNA damage by the mitotic spindle. Proc Natl Acad Sci United states of america 2010, 107: 4159–4164. x.1073/pnas.0912143106
-
Bignell GR, Greenman CD, Davies H, Butler AP, Edkins S, Andrews JM, Buck G, Chen Fifty, Beare D, Latimer C, Widaa South, Hinton J, Fahey C, Fu B, Swamy S, Dalgliesh GL, Teh BT, Deloukas P, Yang F, Campbell PJ, Futreal PA, Stratton MR: Signatures of mutation and selection in the cancer genome. Nature 2010, 463: 893–898. 10.1038/nature08768
-
Beroukhim R, Mermel CH, Porter D, Wei G, Raychaudhuri S, Donovan J, Barretina J, Boehm JS, Dobson J, Urashima Thousand, Mc Henry One thousand, Pinchback RM, Ligon AH, Cho YJ, Haery 50, Greulich H, Reich M, Winckler Westward, Lawrence MS, Weir BA, Tanaka KE, Chiang DY, Bass AJ, Loo A, Hoffman C, Prensner J, Liefeld T, Gao Q, Yecies D, Signoretti S, et al.: The landscape of somatic re-create-number alteration beyond human cancers. Nature 2010, 463: 899–905. 10.1038/nature08822
-
Draviam VM, Xie S, Sorger PK: Chromosome segregation and genomic stability. Curr Opin Genet Dev 2004, fourteen: 120–125. 10.1016/j.gde.2004.02.007
-
Martínez-A C, van Wely KH: Are aneuploidy and chromosome breakage caused by a CINgle machinery? Jail cell Cycle 2010, 9: 12.
-
McClintock B: The Stability of Broken Ends of Chromosomes in Zea Mays. Genetics 1941, 26: 234–282.
-
Schrock Due east, Blume C, Meffert MC, du Manoir S, Bersch Westward, Kiessling M, Lozanowa T, Thiel Yard, Witkowski R, Ried T, Cremer T: Recurrent gain of chromosome arm 7q in low-class astrocytic tumors studied by comparative genomic hybridization. Genes Chromosomes Cancer 1996, 15: 199–205. x.1002/(SICI)1098-2264(199604)15:4<199::AID-GCC1>3.0.CO;2-X
-
Roylance R, Gorman P, Papior T, Wan YL, Ives Yard, Watson JE, Collins C, Wortham N, Langford C, Fiegler H, Carter Due north, Gillett C, Sasieni P, Pinder Due south, Hanby A, Tomlinson I: A comprehensive report of chromosome 16q in invasive ductal and lobular chest carcinoma using array CGH. Oncogene 2006, 25: 6544–6553. 10.1038/sj.onc.1209659
-
Rupa DS, Hasegawa Fifty, Eastmond DA: Detection of chromosomal breakage in the 1cen-1q12 region of interphase human lymphocytes using multicolor fluorescence in situ hybridization with tandem Dna probes. Cancer Res 1995, 55: 640–645.
-
Wurster DH, Benirschke K: Indian muntjac, Muntiacus muntjak : a deer with a low diploid chromosome number. Science 1970, 168: 1364–1366. 10.1126/science.168.3937.1364
-
Walen KH, Chocolate-brown SW: Chromosomes in a marsupial ( Potorous tridactylis ) tissue culture. Nature 1962, 194: 406. 10.1038/194406a0
-
Toder R, O'Neill RJ, Wienberg J, O'Brien PC, Voullaire Fifty, Marshall-Graves JA: Comparative chromosome painting between two marsupials: origins of an Xx/XY1Y2 sex chromosome system. Mamm Genome 1997, eight: 418–422. 10.1007/s003359900459
-
Cimini D, Cameron LA, Salmon ED: Anaphase spindle mechanics prevent mis-segregation of merotelically oriented chromosomes. Curr Biol 2004, 14: 2149–2155. x.1016/j.cub.2004.11.029
-
Rajagopalan H, Lengauer C: Aneuploidy and cancer. Nature 2004, 432: 338–341. 10.1038/nature03099
-
Vig BK, Henderson A: Aneuploidy in male Indian muntjac cells is express to the Y2 chromosome. Mutagenesis 1998, xiii: 33–37. 10.1093/mutage/13.1.33
-
Dotiwala F, Harrison JC, Jain S, Sugawara Northward, Haber JE: Mad2 Prolongs Dna Damage Checkpoint Arrest Acquired past a Double-Strand Break via a Centromere-Dependent Machinery. Curr Biol 2010, twenty: 328–332. 10.1016/j.cub.2009.12.033
-
Fang Y, Liu T, Wang X, Yang YM, Deng H, Kunicki J, Traganos F, Darzynkiewicz Z, Lu 50, Dai Due west: BubR1 is involved in regulation of DNA damage responses. Oncogene 2006, 25: 3598–3605. x.1038/sj.onc.1209392
-
Mikhailov A, Cole RW, Rieder CL: DNA damage during mitosis in man cells delays the metaphase/anaphase transition via the spindle-assembly checkpoint. Curr Biol 2002, 12: 1797–1806. 10.1016/S0960-9822(02)01226-5
-
Touil N, Elhajouji A, Thierens H, Kirsch-Volders M: Analysis of chromosome loss and chromosome segregation in cytokinesis-blocked human lymphocytes: non-disjunction is the prevalent fault in chromosome segregation produced past low dose exposure to ionizing radiations. Mutagenesis 2000, 15: 1–7. 10.1093/mutage/xv.1.i
-
Kirsch-Volders M, Tallon I, Tanzarella C, Sgura A, Hermine T, Parry EM, Parry JM: Mitotic non-disjunction every bit a machinery for in vitro aneuploidy consecration by X-rays in primary human cells. Mutagenesis 1996, xi: 307–313. x.1093/mutage/xi.4.307
-
Sgura A, Antoccia A, Cherubini R, Tanzarella C: Chromosome nondisjunction and loss induced by protons and X rays in primary human being fibroblasts: role of centromeres in aneuploidy. Radiat Res 2001, 156: 225–231. 10.1667/0033-7587(2001)156[0225:CNALIB]2.0.CO;two
-
Bernstein C, Bernstein H, Payne CM, Garewal H: DNA repair/pro-apoptotic dual-role proteins in five major Deoxyribonucleic acid repair pathways: fail-prophylactic protection confronting carcinogenesis. Mutat Res 2002, 511: 145–178. 10.1016/S1383-5742(02)00009-1
-
Rothkamm G, Kruger I, Thompson LH, Lobrich Chiliad: Pathways of Dna double-strand break repair during the mammalian cell cycle. Mol Prison cell Biol 2003, 23: 5706–5715. ten.1128/MCB.23.16.5706-5715.2003
-
Rief N, Lobrich M: Efficient rejoining of radiations-induced DNA double-strand breaks in centromeric DNA of human cells. J Biol Chem 2002, 277: 20572–20582. ten.1074/jbc.M200265200
-
Taccioli GE, Amatucci AG, Beamish HJ, Gell D, Xiang XH, Torres Arzayus MI, Priestley A, Jackson SP, Marshak Rothstein A, Jeggo PA, Herrera VL: Targeted disruption of the catalytic subunit of the Dna-PK gene in mice confers severe combined immunodeficiency and radiosensitivity. Immunity 1998, 9: 355–366. ten.1016/S1074-7613(00)80618-4
-
Barlow C, Hirotsune S, Paylor R, Liyanage M, Eckhaus K, Collins F, Shiloh Y, Crawley JN, Ried T, Tagle D, Wynshaw-Boris A: Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 1996, 86: 159–171. ten.1016/S0092-8674(00)80086-0
-
Strong T, O'Driscoll M, Rief N, Iwabuchi K, Lobrich M, Jeggo PA: ATM and DNA-PK function redundantly to phosphorylate H2AX after exposure to ionizing radiations. Cancer Res 2004, 64: 2390–2396. ten.1158/0008-5472.CAN-03-3207
-
Suzuki Thou, Okada H, Yamauchi 1000, Oka Y, Kodama S, Watanabe M: Qualitative and quantitative analysis of phosphorylated ATM foci induced past low-dose ionizing radiation. Radiat Res 2006, 165: 499–504. 10.1667/RR3542.1
-
Gaymes TJ, Mufti GJ, Rassool FV: Myeloid leukemias accept increased activity of the nonhomologous end-joining pathway and concomitant DNA misrepair that is dependent on the Ku70/86 heterodimer. Cancer Res 2002, 62: 2791–2797.
-
Hosoi Y, Watanabe T, Nakagawa K, Matsumoto Y, Enomoto A, Morita A, Nagawa H, Suzuki Due north: Upward-regulation of Dna-dependent protein kinase action and Sp1 in colorectal cancer. Int J Oncol 2004, 25: 461–468.
-
Pucci South, Mazzarelli P, Rabitti C, Giai M, Gallucci M, Flammia 1000, Alcini A, Altomare 5, Fazio VM: Tumor specific modulation of KU70/80 DNA binding activeness in chest and bladder homo tumor biopsies. Oncogene 2001, 20: 739–747. ten.1038/sj.onc.1204148
-
Collis SJ, DeWeese TL, Jeggo PA, Parker AR: The life and death of Deoxyribonucleic acid-PK. Oncogene 2005, 24: 949–961. x.1038/sj.onc.1208332
-
Secretan MB, Scuric Z, Oshima J, Bishop AJ, Howlett NG, Yau D, Schiestl RH: Outcome of Ku86 and Dna-PKcs deficiency on non-homologous end-joining and homologous recombination using a transient transfection assay. Mutat Res 2004, 554: 351–364.
-
Iliakis K, Wang H, Perrault AR, Boecker W, Rosidi B, Windhofer F, Wu West, Guan J, Terzoudi One thousand, Pantelias G: Mechanisms of Deoxyribonucleic acid double strand suspension repair and chromosome aberration formation. Cytogenet Genome Res 2004, 104: 14–twenty. 10.1159/000077461
-
Tzung TY, Runger TM: Reduced joining of Dna double strand breaks with an aberrant mutation spectrum in rodent mutants of Dna-PKcs and Ku80. Int J Radiat Biol 1998, 73: 469–474. 10.1080/095530098142004
-
Trachana V, van Wely KH, Guerrero AA, Futterer A, Martínez-A C: Dido disruption leads to centrosome distension and mitotic checkpoint defects compromising chromosome stability. Proc Natl Acad Sci U.s.a. 2007, 104: 2691–2696. 10.1073/pnas.0611132104
-
Chen Southward, Inamdar KV, Pfeiffer P, Feldmann E, Hannah MF, Yu Y, Lee JW, Zhou T, Lees-Miller SP, Povirk LF: Accurate in vitro end joining of a Dna double strand interruption with partially cohesive 3'-overhangs and 3'-phosphoglycolate termini: result of Ku on repair fidelity. J Biol Chem 2001, 276: 24323–24330. 10.1074/jbc.M010544200
-
Cheong North, Perrault AR, Wang H, Wachsberger P, Mammen P, Jackson I, Iliakis K: DNA-PK-independent rejoining of DNA double-strand breaks in human being cell extracts in vitro . Int J Radiat Biol 1999, 75: 67–81. ten.1080/095530099140825
-
Bartek J, Lukas J: DNA harm checkpoints: from initiation to recovery or adaptation. Curr Opin Cell Biol 2007, nineteen: 238–245. 10.1016/j.ceb.2007.02.009
Acknowledgements
The authors thank Dr. Maria Blasco for Ku80 and Dna-PKcs mutant mice, and Catherine Mark for editorial aid. The publication costs for this manuscript were financed by grant PS09/00572 (Fondo de Investigación en Salud) and the experimental work by grant Southward-BIO-0189-2006 (Comunidad Autonoma de Madrid). The Department of Immunology and Oncology was founded and is supported by the Spanish National Research Council (CSIC) and by Pfizer.
Author information
Affiliations
Corresponding author
Additional information
Competing interests
The authors declare that they have no competing interests.
Authors' contributions
AAG performed experiments and analyzed data, CMA designed experiments, KvW wrote the newspaper. All authors read and approved the manuscript.
Authors' original submitted files for images
Rights and permissions
Open Access This article is published nether license to BioMed Central Ltd. This is an Open Access commodity is distributed nether the terms of the Creative Commons Attribution License ( https://creativecommons.org/licenses/by/2.0 ), which permits unrestricted use, distribution, and reproduction in any medium, provided the original work is properly cited.
Reprints and Permissions
Almost this article
Cite this article
Guerrero, A.A., Martínez-A, C. & van Wely, Grand.H. Merotelic attachments and non-homologous end joining are the basis of chromosomal instability. Cell Div 5, thirteen (2010). https://doi.org/10.1186/1747-1028-5-thirteen
-
Received:
-
Accepted:
-
Published:
-
DOI : https://doi.org/10.1186/1747-1028-5-13
Keywords
- Chromosome Segregation
- Chromosomal Instability
- Chromosome Breakage
- Spindle Checkpoint
- Mitotic Checkpoint
Source: https://celldiv.biomedcentral.com/articles/10.1186/1747-1028-5-13
0 Response to "What would happen if a chromosome failed to attach to spindle fibers?"
Post a Comment